Chemistry, 14.11.2020 19:40 ggdvj9gggsc
A steel tank contains carbon dioxide at a pressure of 13.0 atm when the temperature is 34oC. What will be the internal gas pressure when the tank and its contents are heated to 100oC


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
A steel tank contains carbon dioxide at a pressure of 13.0 atm when the temperature is 34oC. What wi...
Questions

Mathematics, 27.07.2021 03:10


Social Studies, 27.07.2021 03:10

Mathematics, 27.07.2021 03:10



Mathematics, 27.07.2021 03:10


Spanish, 27.07.2021 03:10







Mathematics, 27.07.2021 03:10


Mathematics, 27.07.2021 03:30


History, 27.07.2021 03:30

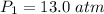
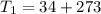
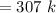
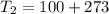
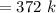
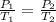
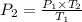
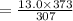
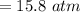
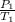 =
= 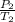
 =
=