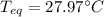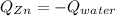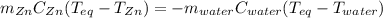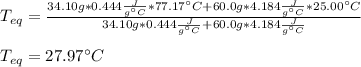Chemistry, 15.11.2020 01:00 george6871
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water shown in the interactive.
Calculate the final temperature of the water. The specific heat of nickel is 0.444 J/g °C and the specific heat of water is
4.184 J/g °C.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Why do you suppose the structural polysaccharide cellulose does not contain branches? why do you suppose the structural polysaccharide cellulose does not contain branches? branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby decreasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby increasing the rigidity and strength of the microfibrils. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into globules, thereby increasing the flexibility and strength of the globules. branches in the molecule would generate side chains that would almost certainly make it difficult to pack the cellulose molecules into microfibrils, thereby decreasing the rigidity and strength of the microfibrils.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water show...
Questions


Mathematics, 10.07.2019 05:30



Mathematics, 10.07.2019 05:30

Mathematics, 10.07.2019 05:30



Mathematics, 10.07.2019 05:30







Biology, 10.07.2019 05:30

Mathematics, 10.07.2019 05:30

Biology, 10.07.2019 05:30

Biology, 10.07.2019 05:30