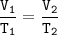Chemistry, 15.11.2020 20:10 queenkimm26
A balloon contains 3300 mL of helium gas at 63 °C. What is the final volume, in milliliters, of the gas when the temperature changes to each of the following, if pressure and amount of gas do not change? Part A 78 C Express your answer to two significant figures and include the appropriate units. UA Vi= Value Units

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
A balloon contains 3300 mL of helium gas at 63 °C. What is the final volume, in milliliters, of the...
Questions

Mathematics, 12.02.2020 10:39



Computers and Technology, 12.02.2020 10:39






Mathematics, 12.02.2020 10:42




Biology, 12.02.2020 10:43

Mathematics, 12.02.2020 10:44




Biology, 12.02.2020 10:57

History, 12.02.2020 10:57