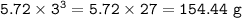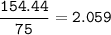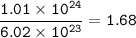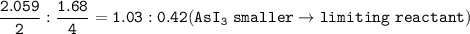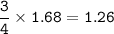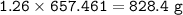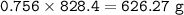The compound As2I4 is synthesized by reaction of arsenic metal with arsenic triiodide. If a solid cubic block of arsenic (d = 5.72 g/cm3 ) that is 3.00 cm on edge is allowed to react with 1.01 * 10^24 molecules of arsenic triiodide, how much As2I4 can be prepared? If the percent yield of As2I4 was 75.6%, what mass of As2I4 was actually isolated?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
The compound As2I4 is synthesized by reaction of arsenic metal with arsenic triiodide. If a solid cu...
Questions



Computers and Technology, 02.03.2020 21:19


Advanced Placement (AP), 02.03.2020 21:19



Biology, 02.03.2020 21:20

English, 02.03.2020 21:20





Biology, 02.03.2020 21:20

Mathematics, 02.03.2020 21:20





Mathematics, 02.03.2020 21:20