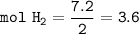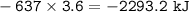Chemistry, 15.11.2020 20:40 calistaallen1734
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g) + H2O (g) △H = −637 kJ
1) Balance the chemical reaction.
2) Identify this reaction as endothermic or exothermic.
3) Calculate how much heat is released when 7.20 g of H2 reacts in this situation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1
You know the right answer?
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g)...
Questions

Mathematics, 09.12.2020 06:20

Computers and Technology, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20

History, 09.12.2020 06:20



Mathematics, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20






Mathematics, 09.12.2020 06:20

Mathematics, 09.12.2020 06:20


Mathematics, 09.12.2020 06:20


Social Studies, 09.12.2020 06:20