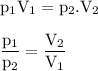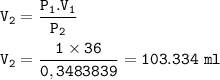Chemistry, 15.11.2020 23:10 gildedav001
If the pressure on 36.0 mL of a gas at STP is changed to a pressure of 35.3 kPa at constant temperature, the new volume of the gas is:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 16:00
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a) 3.4 mol h2so4 b) 6.8 mol h2so4 c) 10.2 mol h2so4 d) 13.6 mol h2so4 a) 3.4 mol h2so4
Answers: 1
You know the right answer?
If the pressure on 36.0 mL of a gas at STP is changed to a pressure of 35.3 kPa at constant temperat...
Questions




Mathematics, 25.09.2020 21:01






English, 25.09.2020 21:01


English, 25.09.2020 21:01


History, 25.09.2020 21:01

Biology, 25.09.2020 21:01

Mathematics, 25.09.2020 21:01

Mathematics, 25.09.2020 21:01



Physics, 25.09.2020 21:01