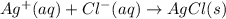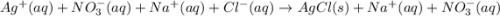Chemistry, 16.11.2020 05:20 Jennifer16253
Enter the balanced NET IONIC equation for the potentially unbalanced equation AgNO3(aq)+NaCl(aq)→AgCl(s)+NaNO3(aq ).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1


Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Enter the balanced NET IONIC equation for the potentially unbalanced equation AgNO3(aq)+NaCl(aq)→AgC...
Questions

Mathematics, 01.04.2020 22:36




Mathematics, 01.04.2020 22:36

Mathematics, 01.04.2020 22:36


Mathematics, 01.04.2020 22:36




Mathematics, 01.04.2020 22:37


History, 01.04.2020 22:37


Mathematics, 01.04.2020 22:37


Arts, 01.04.2020 22:37