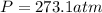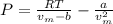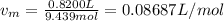Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1



Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
A 9.439 mol sample of oxygen gas is maintained in a 0.8200 L container at 304.4 K. What is the press...
Questions

Business, 02.03.2021 06:20



Geography, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20

Biology, 02.03.2021 06:20


Mathematics, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20



Mathematics, 02.03.2021 06:20



Mathematics, 02.03.2021 06:20


Biology, 02.03.2021 06:20

Chemistry, 02.03.2021 06:20

Mathematics, 02.03.2021 06:20