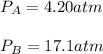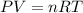Chemistry, 16.11.2020 17:00 noglapotato
A 7.12 L cylinder contains 1.21 mol of gas A and 4.94 mol of gas B, at a temperature of 28.1 °C. Calculate the partial pressure of each gas in the cylinder. Assume ideal gas behavior.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
A 7.12 L cylinder contains 1.21 mol of gas A and 4.94 mol of gas B, at a temperature of 28.1 °C. Cal...
Questions



Mathematics, 04.12.2021 03:40

Mathematics, 04.12.2021 03:40

Arts, 04.12.2021 03:40



Mathematics, 04.12.2021 03:40

Mathematics, 04.12.2021 03:40



Mathematics, 04.12.2021 03:40


Biology, 04.12.2021 03:40




Mathematics, 04.12.2021 03:40


History, 04.12.2021 03:40