
Chemistry, 17.11.2020 01:00 JvGaming2001
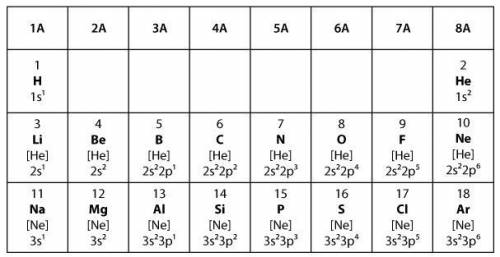
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. Ionic bonds form when one element donates one or more electrons to another element to make a complete outer shell of electrons.
Which statement best explains the type of bond that will form between two elements from group 6A in the model?
A
The two elements will form a covalent bond because both elements will share a single electron in order to have full outer shells.
B
The two elements will form a covalent bond because both elements will share a pair of electrons in order to have full outer shells.
C
The two elements will form an ionic bond because one of the elements will donate one electron to the other element in order to have full outer shells.
D
The two elements will form an ionic bond because one of the elements will donate two electrons to the other element in order to have full outer shells.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. I...
Questions


Mathematics, 11.09.2021 01:30



Business, 11.09.2021 01:30

Mathematics, 11.09.2021 01:30




Mathematics, 11.09.2021 01:30



Mathematics, 11.09.2021 01:30


Chemistry, 11.09.2021 01:30

Social Studies, 11.09.2021 01:30



History, 11.09.2021 01:30




