
Chemistry, 17.11.2020 03:10 shanicar33500
When 0.20 M NH4Cl (aq) and 0.20 M NaOH(aq) are mixed, the reaction represented by the equation above occurs and a strong smell of ammonia, NH3 is observed. Based on this information, which of the following statements is true?
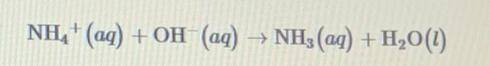

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

You know the right answer?
When 0.20 M NH4Cl (aq) and 0.20 M NaOH(aq) are mixed, the reaction represented by the equation above...
Questions

Mathematics, 01.01.2020 14:31

Biology, 01.01.2020 14:31

Mathematics, 01.01.2020 14:31

Biology, 01.01.2020 14:31


Geography, 01.01.2020 14:31

Mathematics, 01.01.2020 14:31

Computers and Technology, 01.01.2020 14:31


English, 01.01.2020 14:31





Spanish, 01.01.2020 14:31



Mathematics, 01.01.2020 14:31




