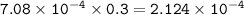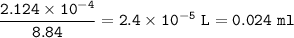Chemistry, 17.11.2020 06:30 jrfranckowiak
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15...
Questions


Geography, 10.12.2019 03:31

Mathematics, 10.12.2019 03:31


Chemistry, 10.12.2019 03:31

Mathematics, 10.12.2019 03:31

French, 10.12.2019 03:31


Mathematics, 10.12.2019 03:31


Mathematics, 10.12.2019 03:31


Mathematics, 10.12.2019 03:31

Advanced Placement (AP), 10.12.2019 03:31

Mathematics, 10.12.2019 03:31



English, 10.12.2019 03:31


![\tt [H^+]=10^{-3.15}=7.08\times 10^{-4}](/tpl/images/0903/9462/83b78.png)