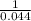19. The reaction 2NOBr (g) → 2 NO (g) + Br2 (g) is a second-order reaction with a rate constant of 0.80 M-1s-1 at 11 °C. If the initial concentration of NOBr is 0.0440 M, the concentration of NOBr after 6.0 seconds is . A) 0.0276 M B) 0.0324 M C) 0.0363 M D) 0.0348 M E) 0.0402 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
19. The reaction 2NOBr (g) → 2 NO (g) + Br2 (g) is a second-order reaction with a rate constant of 0...
Questions

English, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31

Chemistry, 04.12.2019 06:31



History, 04.12.2019 06:31

Social Studies, 04.12.2019 06:31

Health, 04.12.2019 06:31


Mathematics, 04.12.2019 06:31

Chemistry, 04.12.2019 06:31

Chemistry, 04.12.2019 06:31


History, 04.12.2019 06:31

Business, 04.12.2019 06:31

Mathematics, 04.12.2019 06:31



History, 04.12.2019 06:31

World Languages, 04.12.2019 06:31

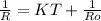

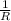 = 0.8*6+
= 0.8*6+