
Chemistry, 17.11.2020 17:30 rodriguezbrian050702
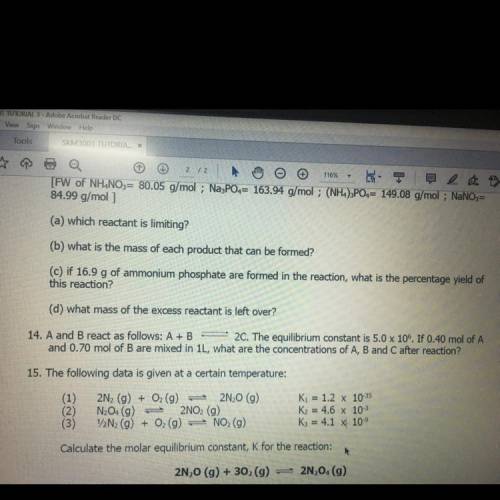
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A and 0.70 mol of B are mixed in 1L, what are the concentrations of A, B and C after reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A
and 0...
Questions

Mathematics, 03.02.2021 20:50

Business, 03.02.2021 20:50

Arts, 03.02.2021 20:50



Physics, 03.02.2021 20:50

Mathematics, 03.02.2021 20:50

Mathematics, 03.02.2021 20:50



Mathematics, 03.02.2021 20:50



History, 03.02.2021 20:50

Mathematics, 03.02.2021 20:50

Mathematics, 03.02.2021 20:50


Mathematics, 03.02.2021 20:50

Mathematics, 03.02.2021 20:50



