Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
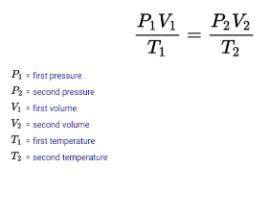
STP= Standard temperature and pressure . standard temperature is 273K and standard pressure is 760 M...
Questions




Mathematics, 16.12.2020 17:20



Mathematics, 16.12.2020 17:20

Mathematics, 16.12.2020 17:20

Mathematics, 16.12.2020 17:20

Mathematics, 16.12.2020 17:20

History, 16.12.2020 17:20

Physics, 16.12.2020 17:20

English, 16.12.2020 17:20

Mathematics, 16.12.2020 17:20


History, 16.12.2020 17:20



Mathematics, 16.12.2020 17:20