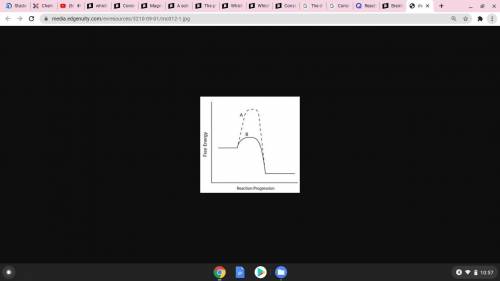
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Questions

Mathematics, 05.05.2021 20:50

English, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50


History, 05.05.2021 20:50

Chemistry, 05.05.2021 20:50





Mathematics, 05.05.2021 20:50

History, 05.05.2021 20:50

Mathematics, 05.05.2021 20:50

History, 05.05.2021 20:50


Chemistry, 05.05.2021 20:50



Social Studies, 05.05.2021 20:50

Chemistry, 05.05.2021 20:50