Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
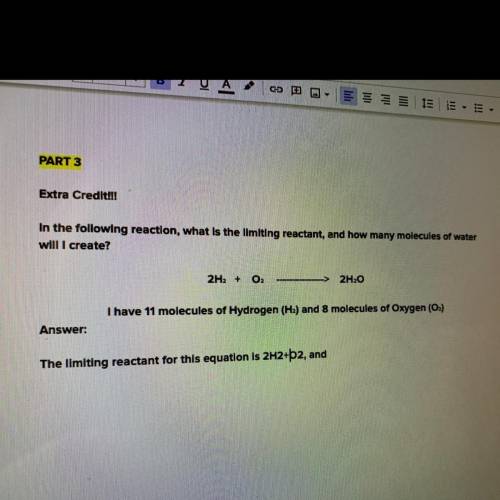
In the following reaction, what is the limiting reactant, and how many molecules of water will I cre...
Questions


Chemistry, 27.08.2019 18:00

Computers and Technology, 27.08.2019 18:00

Mathematics, 27.08.2019 18:00


History, 27.08.2019 18:00



Mathematics, 27.08.2019 18:00


Social Studies, 27.08.2019 18:00


Biology, 27.08.2019 18:00

Social Studies, 27.08.2019 18:00