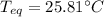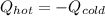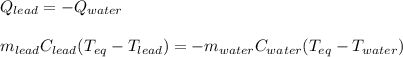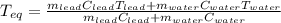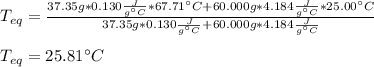Chemistry, 18.11.2020 02:40 diamondk2019
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown in the interactive. Calculate the final temperature of the water. The specific heat of lead is 0.130 J/g⋅°C and the specific heat of water is 4.184 J/g⋅°C .
mass of water: 60.000g
initial temperature of water: 25.00 C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
A chemist heats 37.35 g of lead to 67.71 °C , then places the metal sample in the cup of water shown...
Questions

Mathematics, 23.04.2021 01:00

English, 23.04.2021 01:00

Biology, 23.04.2021 01:00

English, 23.04.2021 01:00



Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00

Geography, 23.04.2021 01:00


Mathematics, 23.04.2021 01:00

Mathematics, 23.04.2021 01:00



Biology, 23.04.2021 01:00



Spanish, 23.04.2021 01:00

English, 23.04.2021 01:00