
Chemistry, 18.11.2020 03:20 teamroper35
The Case of the Missing Mass: Students have completed the experiment of combining vinegar and baking soda. They notice that their beginning mass in the video does not match the ending one. Use the equation(below) and the Law of Conservation of Mass to explain why the students have NOT destroyed atoms in their experiment. This is the chemical equation for the experiment:
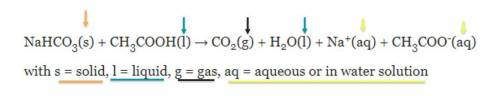

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
The Case of the Missing Mass: Students have completed the experiment of combining vinegar and baking...
Questions

Mathematics, 22.02.2021 20:40

English, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40



English, 22.02.2021 20:40

Physics, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40

History, 22.02.2021 20:40

Mathematics, 22.02.2021 20:40








Computers and Technology, 22.02.2021 20:40



