
Chemistry, 18.11.2020 07:50 swaggernas
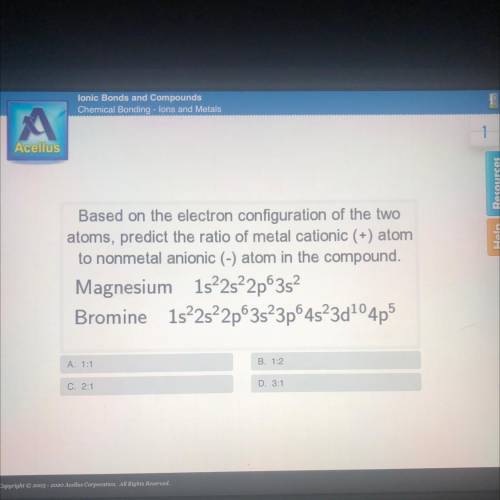
Based on the electron configuration of the two
atoms, predict the ratio of metal cationic (+) atom
to nonmetal anionic (-) atom in the compound.
Magnesium 1s22s22p3s
Bromine 1522s22p63s23p64523d104p5
A. 1:1
B. 1:2
C. 2:1
D. 3:1

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
Based on the electron configuration of the two
atoms, predict the ratio of metal cationic (+) atom<...
Questions

Mathematics, 17.07.2019 13:10



Computers and Technology, 17.07.2019 13:10


Biology, 17.07.2019 13:10

Physics, 17.07.2019 13:10



Advanced Placement (AP), 17.07.2019 13:10

Mathematics, 17.07.2019 13:10


Mathematics, 17.07.2019 13:20


Mathematics, 17.07.2019 13:20

History, 17.07.2019 13:20

Mathematics, 17.07.2019 13:20

Mathematics, 17.07.2019 13:20

Mathematics, 17.07.2019 13:20



