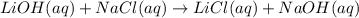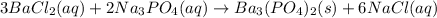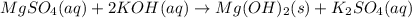Based on the results from this experiment, predict whether some of the following combinations of compounds would precipitate or stay in solution. Explain how you determined that a certain compound should precipitate or be soluble. a. LiOH NaCl b. BaCl2 Na3PO4 c. MgSO4 KOH

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

You know the right answer?
Based on the results from this experiment, predict whether some of the following combinations of com...
Questions






Mathematics, 15.04.2020 01:25

Chemistry, 15.04.2020 01:25


English, 15.04.2020 01:25








Computers and Technology, 15.04.2020 01:25



Mathematics, 15.04.2020 01:25