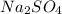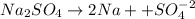Chemistry, 18.11.2020 16:50 kaytlynnmeyer
If a 3.0 M solution of glucose (C6H12O6), a 2.0 M solution of Na2SO4 and a 1.0 M solution of (NH4)3PO4, is made, which solution will have the lowest vapor pressure, highest boiling point, and lowest freezing point?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
If a 3.0 M solution of glucose (C6H12O6), a 2.0 M solution of Na2SO4 and a 1.0 M solution of (NH4)3P...
Questions

Mathematics, 09.11.2020 07:20

Chemistry, 09.11.2020 07:20


Social Studies, 09.11.2020 07:20

Mathematics, 09.11.2020 07:20


Mathematics, 09.11.2020 07:20

Geography, 09.11.2020 07:20

History, 09.11.2020 07:20


Social Studies, 09.11.2020 07:20




Advanced Placement (AP), 09.11.2020 07:20

Chemistry, 09.11.2020 07:20


Computers and Technology, 09.11.2020 07:20


Chemistry, 09.11.2020 07:20