

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equi...
Questions

Biology, 25.05.2021 19:50




Mathematics, 25.05.2021 19:50



Mathematics, 25.05.2021 19:50





Mathematics, 25.05.2021 19:50

Chemistry, 25.05.2021 19:50




Physics, 25.05.2021 19:50

Mathematics, 25.05.2021 19:50

Mathematics, 25.05.2021 19:50

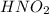
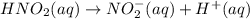
![k_a = \frac{[NO_{2}^{-}][H^{+}]}{HNO_{2}}](/tpl/images/0908/7640/67909.png)


