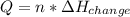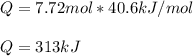Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2


Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
Calculate the quantity of energy required to change 7.72 mol of liquid water to steam at 100oC. The...
Questions

Social Studies, 01.02.2022 14:20



Mathematics, 01.02.2022 14:20


Biology, 01.02.2022 14:20

Mathematics, 01.02.2022 14:20

History, 01.02.2022 14:20

English, 01.02.2022 14:20

History, 01.02.2022 14:20

Computers and Technology, 01.02.2022 14:20

Mathematics, 01.02.2022 14:20


Mathematics, 01.02.2022 14:30



Mathematics, 01.02.2022 14:30

Mathematics, 01.02.2022 14:30


Mathematics, 01.02.2022 14:30