Which of the following shows that the combustion of methane produces 802 kJ/mol of energy?
...

Chemistry, 19.11.2020 14:00 natjojo0512
Which of the following shows that the combustion of methane produces 802 kJ/mol of energy?
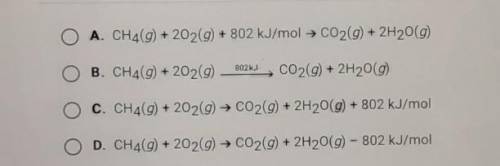

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Questions

History, 05.05.2020 16:38




Mathematics, 05.05.2020 16:38

English, 05.05.2020 16:38


English, 05.05.2020 16:38


Mathematics, 05.05.2020 16:38

Chemistry, 05.05.2020 16:38


English, 05.05.2020 16:38

English, 05.05.2020 16:38

Geography, 05.05.2020 16:38



Business, 05.05.2020 16:38




