Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fe...

Chemistry, 17.10.2019 03:30 amberchule9681
Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fecl2(s)+h2s(g)
a reaction mixture initially contains 0.240 mol fes and 0.646 mol hcl.
what amount (in moles) of the excess reactant is left?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
Questions

History, 15.07.2019 17:30








Mathematics, 15.07.2019 17:30

Mathematics, 15.07.2019 17:30


Mathematics, 15.07.2019 17:30



Mathematics, 15.07.2019 17:30





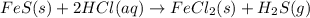
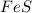 react with 2 mole of
react with 2 mole of 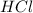
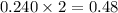 moles of
moles of 


