
Chemistry, 19.11.2020 18:40 natetheman7740
The depictions of ionic compounds do not show distinct compound units but rather a collection of ions in the ratio given by the formula. Explain how the drawing of NaCl both illustrates that the compound exists as a large collection of ions and that the ratio of sodium ions to chloride ions is 1-to-1 as indicated by the formula NaCl.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

You know the right answer?
The depictions of ionic compounds do not show distinct compound units but rather a collection of ion...
Questions


Mathematics, 02.09.2019 09:00


Chemistry, 02.09.2019 09:00


English, 02.09.2019 09:00


English, 02.09.2019 09:00

Physics, 02.09.2019 09:00

Social Studies, 02.09.2019 09:00

Mathematics, 02.09.2019 09:00

Health, 02.09.2019 09:00

Physics, 02.09.2019 09:00


Physics, 02.09.2019 09:00

Mathematics, 02.09.2019 09:00

Social Studies, 02.09.2019 09:00


Business, 02.09.2019 09:00

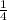 = 4 Na+
= 4 Na+ 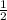 +
+ 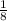 x8 corners = 4Cl-
x8 corners = 4Cl-


