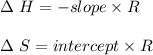A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the solubility of your salt. B. Would this initial evaporation affect the calculated solubility of your salt at each subsequent experimental saturation temperature, or just the initial temperature? Explain.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the so...
Questions

History, 03.06.2021 01:00


Mathematics, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00


English, 03.06.2021 01:00

Business, 03.06.2021 01:00




English, 03.06.2021 01:00



Mathematics, 03.06.2021 01:00

History, 03.06.2021 01:00



Mathematics, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00

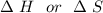 are calculated by
are calculated by 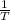 v/s lnKsp
v/s lnKsp