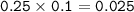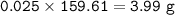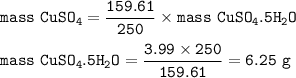Chemistry, 19.11.2020 20:10 Braxtonw875
Approximately what mass of CuSO4*5H20 (250 g mol^-1) is required to prepare 250 mL of 0.10 M copper(ll)
sulfate solution?
A) 4.0 g
B
6.2 g
С
34 g
D
85 g
E
140 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
Approximately what mass of CuSO4*5H20 (250 g mol^-1) is required to prepare 250 mL of 0.10 M copper(...
Questions

Mathematics, 19.05.2021 01:00

English, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00

History, 19.05.2021 01:00




Geography, 19.05.2021 01:00

Chemistry, 19.05.2021 01:00


Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00





Spanish, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00