Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Which statement describes the appearance of a temperature-vs.-time graph? a horizontal line shows that the temperature increases at a constant rate over time. a vertical line shows that the temperature decreases at a constant rate over time. horizontal lines where the temperature is constant during phase changes connect upward-sloping lines where the temperature increases. horizontal lines where the temperature increases are connected by upward-sloping lines where the temperature is constant for each phase.
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
A 100.0 mL sample of 0.18 M HClO4 (Strong acid) is titrated with 0.27 M LiOH. Determine the pH of th...
Questions





Biology, 22.06.2019 08:30

Mathematics, 22.06.2019 08:30

History, 22.06.2019 08:30

Spanish, 22.06.2019 08:30


English, 22.06.2019 08:30




Health, 22.06.2019 08:30






History, 22.06.2019 08:30


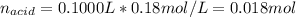
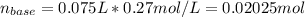
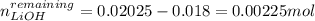
![[LiOH]=\frac{0.00225mol}{0.1750L}=0.013M](/tpl/images/0917/1926/d60d2.png)
![pOH=-log([OH^-])=-log(0.013M)=1.89](/tpl/images/0917/1926/5deb1.png)