
Chemistry, 20.11.2020 17:10 quincyjosiah07
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molecular, medido a TPE, reacciona con un exceso de cloro molecular gaseoso. MHCl = 36.5 g/mol H2(g) + Cl2(g) → 2HCl(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molec...
Questions


Biology, 04.02.2021 21:10


Biology, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

Arts, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

English, 04.02.2021 21:10


Arts, 04.02.2021 21:10

Mathematics, 04.02.2021 21:10

English, 04.02.2021 21:10


Mathematics, 04.02.2021 21:10

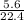 = 0.25mole
= 0.25mole 

