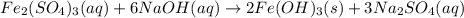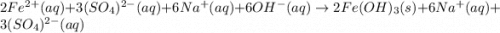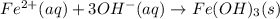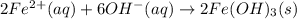Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
An aqueous solution of Iron(III) sulfate, Fe2(SO4)3, is mixed with an aqueous solution of Sodium hyd...
Questions




Mathematics, 03.05.2020 13:04

Mathematics, 03.05.2020 13:04


Mathematics, 03.05.2020 13:04

Business, 03.05.2020 13:04


Mathematics, 03.05.2020 13:04



English, 03.05.2020 13:04


Social Studies, 03.05.2020 13:04

Social Studies, 03.05.2020 13:04


Mathematics, 03.05.2020 13:04

Mathematics, 03.05.2020 13:04

Mathematics, 03.05.2020 13:04