
Chemistry, 20.11.2020 21:10 littlemoneyh
If you start with 1.5 moles of H2O, how many moles of H4SiO4 do you make?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
If you start with 1.5 moles of H2O, how many moles of H4SiO4 do you make?...
Questions




English, 18.11.2020 21:00



Mathematics, 18.11.2020 21:00


Spanish, 18.11.2020 21:00

History, 18.11.2020 21:00

Mathematics, 18.11.2020 21:00

Advanced Placement (AP), 18.11.2020 21:00

Health, 18.11.2020 21:00





Mathematics, 18.11.2020 21:00

History, 18.11.2020 21:00

SAT, 18.11.2020 21:00

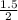 = 0.75moles of H₄SiO₄
= 0.75moles of H₄SiO₄

