

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2


Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
How much tin II fluoride will be made when reacting 45.0 grams of tin with an excess of hydrofluoric...
Questions


History, 19.08.2019 20:00

Mathematics, 19.08.2019 20:00

Mathematics, 19.08.2019 20:00

Mathematics, 19.08.2019 20:00


English, 19.08.2019 20:00

History, 19.08.2019 20:00

Social Studies, 19.08.2019 20:00

Geography, 19.08.2019 20:00






Biology, 19.08.2019 20:00



Mathematics, 19.08.2019 20:00

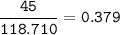
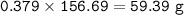 ⇒theoretical
⇒theoretical


