
Chemistry, 21.11.2020 22:20 maxy7347go
The density of ethanol, C 2H 5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produce 25.0 g of CO 2 according to the following chemical equation? C 2H 5OH( l) + 3 O 2( g) → 2 CO 2( g) + 3 H 2O( l)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 21:10
What might the student have done that caused this error? list all possible causes
Answers: 1

Chemistry, 23.06.2019 21:30
If 1.oo mol cs2 reacts with 1.00 mol o2 , identify the limiting reactant
Answers: 1
You know the right answer?
The density of ethanol, C 2H 5OH, is 0.789 g/mL. How many milliliters of ethanol are needed to produ...
Questions

English, 18.12.2020 07:20

Physics, 18.12.2020 07:20

Computers and Technology, 18.12.2020 07:20





Biology, 18.12.2020 07:20



Mathematics, 18.12.2020 07:20


History, 18.12.2020 07:20

Mathematics, 18.12.2020 07:20

English, 18.12.2020 07:20


Mathematics, 18.12.2020 07:30


Mathematics, 18.12.2020 07:30

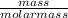
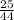 = 0.57mol
= 0.57mol 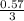 = 0.19mole of ethanol
= 0.19mole of ethanol 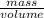
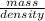
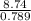 = 11.1mL
= 11.1mL

