
Chemistry, 22.11.2020 05:00 destinyharris8502
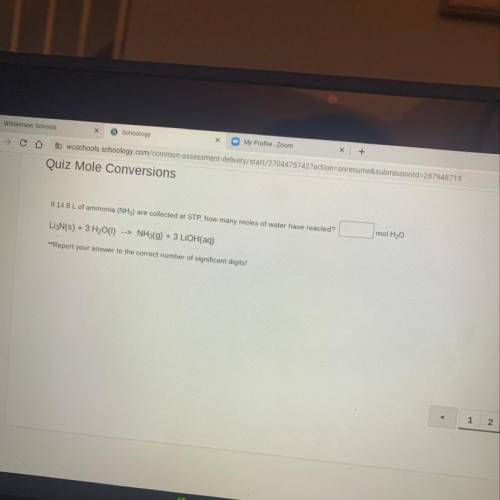
If 14.8 L of ammonia (NH3) are collected at STP, how many moles of water have reacted?
__ mol H20
Li3N(S) + 3 H20(1) --> NH3(g) + 3 LiOH(aq)
**Report your answer to the correct number of significant digits!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1
You know the right answer?
If 14.8 L of ammonia (NH3) are collected at STP, how many moles of water have reacted?
__ mol H20
Questions

English, 23.09.2019 15:30

Health, 23.09.2019 15:30


Mathematics, 23.09.2019 15:30

Biology, 23.09.2019 15:30

Mathematics, 23.09.2019 15:30

Mathematics, 23.09.2019 15:30







Mathematics, 23.09.2019 15:30



Chemistry, 23.09.2019 15:30


Mathematics, 23.09.2019 15:30



