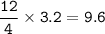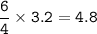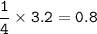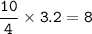Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
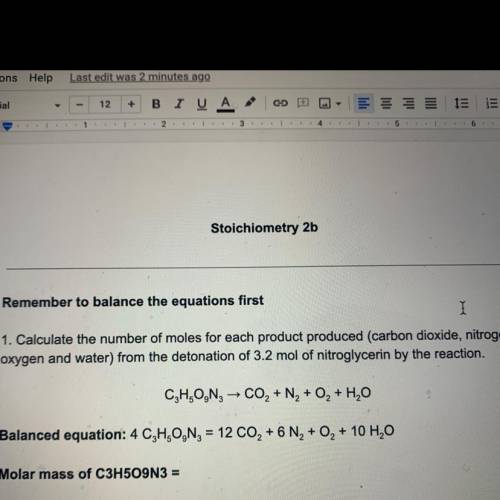
Remember to balance the equations first
1. Calculate the number of moles for each product produced...
Questions

English, 22.06.2021 02:20


Mathematics, 22.06.2021 02:20




Physics, 22.06.2021 02:20

Physics, 22.06.2021 02:20

English, 22.06.2021 02:20





Mathematics, 22.06.2021 02:20





Mathematics, 22.06.2021 02:20

Health, 22.06.2021 02:20