
Chemistry, 23.11.2020 01:20 HarleyQuinn117
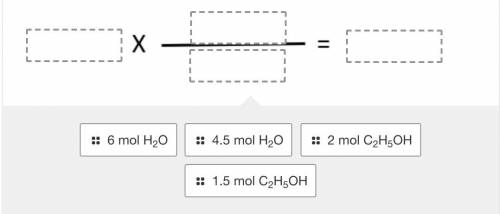
Using the balanced reaction below, drag and drop the terms into the correct location to solve the following problem:
If 1.5 moles of ethanol (C2H5OH) react, how many moles of water will be formed?
2 C2H5OH + 7 O2 --> 4 CO2 + 6 H2O

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2
You know the right answer?
Using the balanced reaction below, drag and drop the terms into the correct location to solve the fo...
Questions

Physics, 27.07.2021 14:30


Mathematics, 27.07.2021 14:30



Computers and Technology, 27.07.2021 14:30


Computers and Technology, 27.07.2021 14:30

Mathematics, 27.07.2021 14:30

Mathematics, 27.07.2021 14:30

Chemistry, 27.07.2021 14:30

Business, 27.07.2021 14:30

Geography, 27.07.2021 14:30

Social Studies, 27.07.2021 14:30

Mathematics, 27.07.2021 14:30


Mathematics, 27.07.2021 14:30

Mathematics, 27.07.2021 14:30





