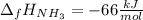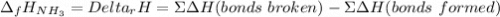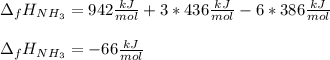Chemistry, 23.11.2020 02:30 afitzgerald
The formation of ammonia is represented by the equation N2(g) + 3H2(g) ⇌ 2NH3(g). Determine the enthalpy of formation of ammonia given the following mean bond enthalpies (kJmol-1): N≡N 942; H-H 436; N-H 386

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
The formation of ammonia is represented by the equation N2(g) + 3H2(g) ⇌ 2NH3(g). Determine the enth...
Questions

Chemistry, 05.02.2021 21:00


Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Computers and Technology, 05.02.2021 21:00


Mathematics, 05.02.2021 21:00

Health, 05.02.2021 21:00






Social Studies, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

History, 05.02.2021 21:00

History, 05.02.2021 21:00