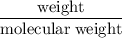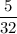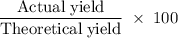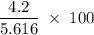Chemistry, 23.11.2020 04:10 corcoranrobert1959
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Please answer the questions in a b c d and e, or at least in any of the ones you know. Thank you.


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 23.06.2019 17:50
The only bonds in a formula unit of caf2 are nonpolar covalent polar covalent ionic metallic
Answers: 1


Chemistry, 23.06.2019 21:30
Acertain substance has a solubility of 12 grams in 100 grams of water at 20°c. this means that the substance will begin to dissolve when 12 grams are present in solution when 12 grams of the substance are stirred into a beaker with 100 g of water, it will begin settling at the bottom when 12 g of the substance are dissolved in 100 grams of water, the solution will be dilute when 12 g of the substance are dissolved in 100 grams of water, the solution will be saturated
Answers: 1
You know the right answer?
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Questions

English, 07.04.2020 16:23



English, 07.04.2020 16:23








Computers and Technology, 07.04.2020 16:23

Computers and Technology, 07.04.2020 16:23



Social Studies, 07.04.2020 16:23




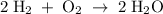
 2
2