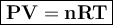Chemistry, 23.11.2020 08:00 brookemcelhaney
0.817 grams of an unknown gas occupies 1.25 L at 27.0 C and a pressure of 765
mm Hg. Is the gas most likely methane (CH 4 ), ethane (C 2 H 6 ), propane or butane?
Show work to support your answer.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 05:30
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table? atom p has an estimated zeff of 7 and is therefore to the left of atom q, which has a zeff of 6. atom p has an estimated zeff of 7 and is therefore to the right of atom q, which has a zeff of 6. atom p has an estimated zeff of 5 and is therefore below atom q, which has a zeff of 4. atom p has an estimated zeff of 5 and is therefore above atom q, which has a zeff of 4.
Answers: 3

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
0.817 grams of an unknown gas occupies 1.25 L at 27.0 C and a pressure of 765
mm Hg. Is the gas mo...
Questions


Spanish, 29.07.2019 17:30



Mathematics, 29.07.2019 17:30




History, 29.07.2019 17:30

Mathematics, 29.07.2019 17:30

Mathematics, 29.07.2019 17:30


Health, 29.07.2019 17:30

Physics, 29.07.2019 17:30



Social Studies, 29.07.2019 17:30


Social Studies, 29.07.2019 17:30

Spanish, 29.07.2019 17:30