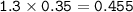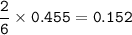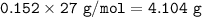What mass of aluminum (in g) would be
required to completely react with 1.30 L of
0.350 M HCl...

Chemistry, 23.11.2020 08:40 amadileaks
What mass of aluminum (in g) would be
required to completely react with 1.30 L of
0.350 M HCl in the following chemical
reaction?
2 Al(s) + 6 HCl(aq) → 2 AICI: (aq) + 3 H2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 12:30
The equilibrium constant kc for the reaction 2 nocl(g) → 2 no(g) + cl2(g) is 0.453 at a certain temperature. a mixture of nocl, no, and cl2 with concentrations 1.30, 1.20, and 0.600 m, respectively, was introduced into a container at this temperature. which of the following is true? 1. no apparent reaction takes place. 2. [cl2] = 0.30 m at equilibrium. 3. nocl(g) is produced until equilibrium is reached. 4. [nocl] = [no] = [cl2] at equilibrium. 5. cl2(g) is produced until equilibrium is
Answers: 3
You know the right answer?
Questions



Mathematics, 09.12.2021 02:00

History, 09.12.2021 02:00


Mathematics, 09.12.2021 02:00



Mathematics, 09.12.2021 02:00



Mathematics, 09.12.2021 02:00


Chemistry, 09.12.2021 02:00




Mathematics, 09.12.2021 02:00

SAT, 09.12.2021 02:00