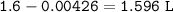Chemistry, 23.11.2020 09:00 Savadt2810
There is a concentrated (12.0 M) solution of HCl in the lab storage area. You need to prepare 1600. mL of HCl solution that has a pH equal to 1.50.
a) What volume of the 12.0 M HCl solution do you need?
b) What volume of water do you need?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

You know the right answer?
There is a concentrated (12.0 M) solution of HCl in the lab storage area. You need to prepare 1600....
Questions



Biology, 29.07.2019 01:30



Biology, 29.07.2019 01:30

Mathematics, 29.07.2019 01:30


Computers and Technology, 29.07.2019 01:30






Health, 29.07.2019 01:30

Geography, 29.07.2019 01:30




History, 29.07.2019 01:30

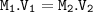
![\tt 10^{-1.5}(pH=-log[H^+])](/tpl/images/0923/0841/24ceb.png) =0.032
=0.032