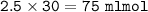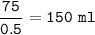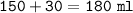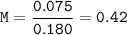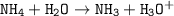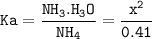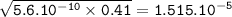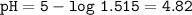Chemistry, 23.11.2020 08:50 raynamg2718
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of NH3 = 1.8 X 10-5
Write the balanced equation for this reaction.
What volume of 0.500 M HCl solution was added?
What is the molarity of the salt produced from the neutralization reaction?
What is the pH of the solution at the equivalence point?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

You know the right answer?
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of N...
Questions

Mathematics, 18.05.2021 21:50

English, 18.05.2021 21:50


Health, 18.05.2021 21:50


Mathematics, 18.05.2021 21:50

Biology, 18.05.2021 21:50



Mathematics, 18.05.2021 21:50



Biology, 18.05.2021 21:50


Mathematics, 18.05.2021 21:50

History, 18.05.2021 21:50

Mathematics, 18.05.2021 21:50

Computers and Technology, 18.05.2021 21:50

Mathematics, 18.05.2021 21:50

English, 18.05.2021 21:50