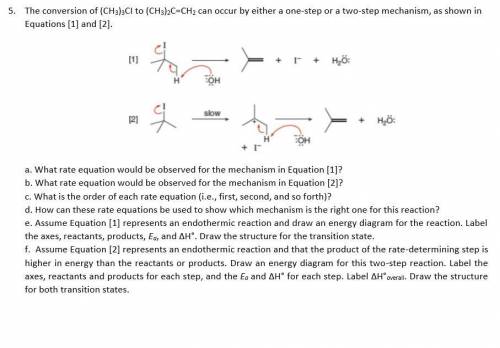
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as shown in
Equations (1) and (2]
[1]
있
+ I + H₂O
HK
OH
[2]
07
Slow
+ H₂O
+ I
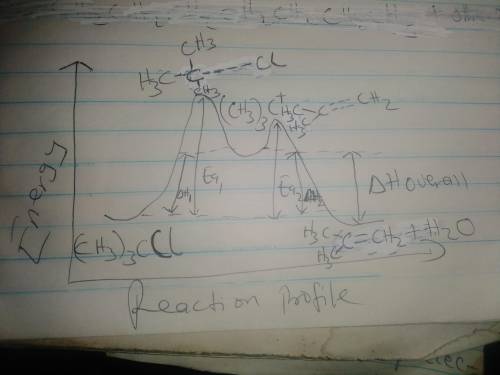
f) Assume Equation [a] represents an endothermic reaction
and that the product of the rate determining step is
higher in energy than the reactants or products.
Draw an energy dagram for this two-step reaction.
Label the axes, reactants and products for each step,
and the Ea and 4to for each step. Label 44° overall.
Draw the structure for both transition states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as sh...
Questions



Mathematics, 28.10.2019 17:31



Mathematics, 28.10.2019 17:31

Geography, 28.10.2019 17:31

Chemistry, 28.10.2019 17:31

Mathematics, 28.10.2019 17:31


English, 28.10.2019 17:31

Chemistry, 28.10.2019 17:31



History, 28.10.2019 17:31


History, 28.10.2019 17:31


Chemistry, 28.10.2019 17:31