Chemistry, 23.11.2020 22:30 buciuceanu8740
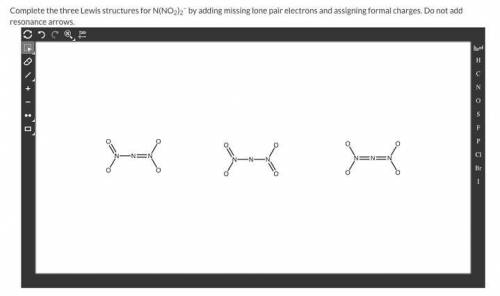
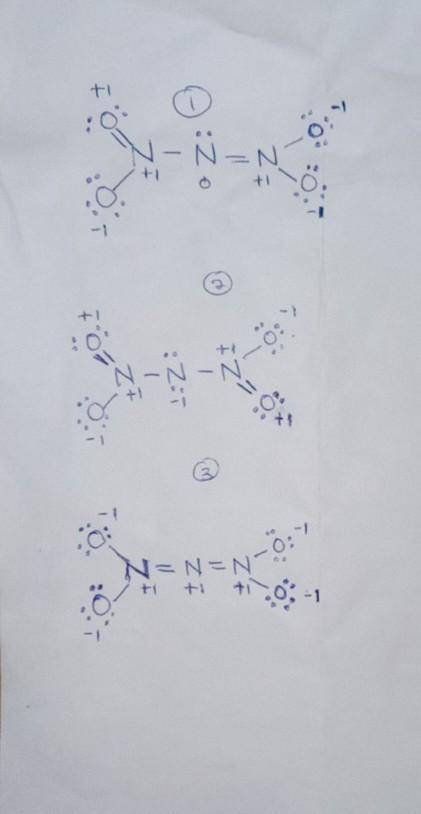
Complete the three Lewis structures for N(NO2)2– by adding missing lone pair electrons and assigning formal charges. Do not add resonance arrow

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

You know the right answer?
Complete the three Lewis structures for N(NO2)2– by adding missing lone pair electrons and assigning...
Questions

Business, 30.09.2019 06:30


Geography, 30.09.2019 06:30

Mathematics, 30.09.2019 06:30

Biology, 30.09.2019 06:30


Mathematics, 30.09.2019 06:30

Mathematics, 30.09.2019 06:30




History, 30.09.2019 06:30




History, 30.09.2019 06:30

History, 30.09.2019 06:30

Mathematics, 30.09.2019 06:30

History, 30.09.2019 06:30

Mathematics, 30.09.2019 06:30