
Chemistry, 24.11.2020 14:00 nagwaelbadawi
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction above.
18 + 25 O2 --> 16 CO2 + 18 H2O
89.6 g
17.3 g
34.5 g
46.2 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction...
Questions

English, 20.05.2021 21:50



English, 20.05.2021 21:50

English, 20.05.2021 21:50

Mathematics, 20.05.2021 21:50

Mathematics, 20.05.2021 21:50



Mathematics, 20.05.2021 21:50


Computers and Technology, 20.05.2021 21:50








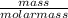
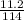 = 0.098mole
= 0.098mole 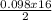 = 0.79mole of CO₂
= 0.79mole of CO₂

