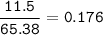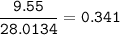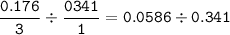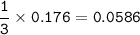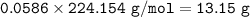Chemistry, 24.11.2020 19:10 natalia9573
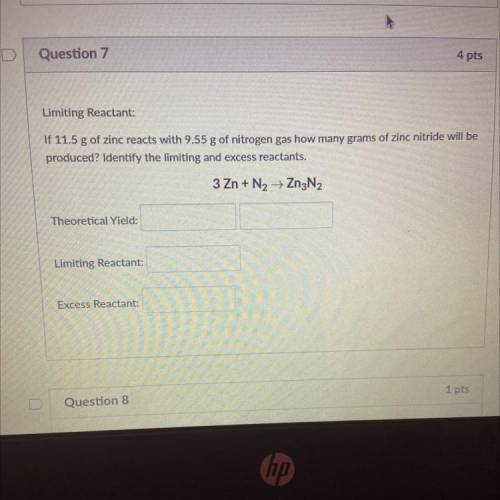
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitride will be
produced? Identify the limiting and excess reactants.
3 Zn + N2 + Zn3N2
Theoretical Yield:
Limiting Reactant:
Excess Reactant:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Limiting Reactant:
If 11.5 g of zinc reacts with 9.55 g of nitrogen gas how many grams of zinc nitr...
Questions



English, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01

Medicine, 13.10.2020 01:01

English, 13.10.2020 01:01






History, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01





Business, 13.10.2020 01:01