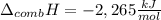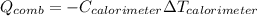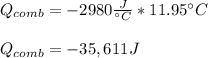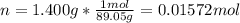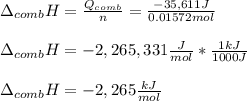Chemistry, 24.11.2020 19:20 hayleegreenwell34
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent complete combustion under constant pressure conditions in a special calorimeter. This calorimeter had a heat capacity of 2980 J °C.1 (Note that the calorimeter was made of a metal shell, a water "substitute" - a special oil, and a thermocouple). The temperature went up by 11.95 degrees.
Required:
Calculate the molar heat of combustion of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent c...
Questions

Mathematics, 05.02.2021 19:50

Mathematics, 05.02.2021 19:50



Mathematics, 05.02.2021 19:50



Mathematics, 05.02.2021 19:50

Mathematics, 05.02.2021 19:50



Chemistry, 05.02.2021 19:50



Mathematics, 05.02.2021 19:50

Physics, 05.02.2021 19:50

Physics, 05.02.2021 19:50

Biology, 05.02.2021 19:50

Health, 05.02.2021 19:50