
Chemistry, 24.11.2020 19:20 melaniem50
The heat capacity of solid iron is 0.447 J/gËC. If the same quantity of energy were transferred to a 450 g chunk of iron at 20ËC, what would be the final temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
The heat capacity of solid iron is 0.447 J/gËC. If the same quantity of energy were transferred to a...
Questions

History, 22.10.2020 16:01

Physics, 22.10.2020 16:01


Computers and Technology, 22.10.2020 16:01

Computers and Technology, 22.10.2020 16:01



Mathematics, 22.10.2020 16:01






Computers and Technology, 22.10.2020 16:01






Mathematics, 22.10.2020 16:01

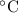 .
.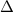 T
T 450 g
450 g 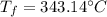
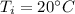
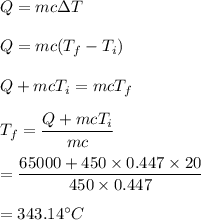
 .
.

